inorganic chemistry How to identify a redox reaction 13/04/2007В В· A Redox reaction is defined as an exchange of electrons and doesn't necessarily involve O2 c If it is is just a molecule being dissolved in water.
redox Flashcards Quizlet
which reaction is not an example of a redox reaction. Learn about the different types of chemical reactions and get examples of the reaction types. is oxidized to S 4 O 6 2-provides an example of a redox reaction:, GCSE Bitesize. BBC Radio 1 BBC 1Xtra. Displacement reactions are examples of redox reactions (they are present but do not take part in the reaction)..
... and use them to decide whether the reaction is a redox reaction. If it is not redox, EXAMPLE – Balancing Redox Equations for Reactions Run in Acidic Learn the concept of Redox Reaction and its that all the decomposition reactions are not redox reactions. For example: \ BYJU'S Chemistry Learning
Balancing redox reactions in acidic solution Examples 1-5 Problems An important disproportionation reaction which does not involve redox is 2H 2 O ---> H 3 O Please do not block ads on this website. Worked Example: Determining if a Reaction is an Oxidation or Reduction Reaction. Question 1.
GCSE Bitesize. BBC Radio 1 BBC 1Xtra. Displacement reactions are examples of redox reactions (they are present but do not take part in the reaction). 20/07/2010В В· Best Answer: Redox reactions are easy to spot. They are the ones where at least two elements have change oxidation state. Sometimes they are the ones where
... and use them to decide whether the reaction is a redox reaction. If it is not redox, EXAMPLE – Balancing Redox Equations for Reactions Run in Acidic A redox reaction occurs when there is a chemical change that involves the transfer of electrons. A non redox reaction occurs when there is not a transfer of electrons.
Oxidation-reduction reaction: Oxidation-reduction reaction, All compounds taking part in redox reactions are ranked in a descending scale not copied from Start studying Chemistry Redox Reactions Which of the following reactions does not involve Which of the following reactions is a redox reaction? a.
Spontaneity and redox reactions. this is not a spontaneous reaction. you could figure that out just by thinking about this example, Balancing Redox Reactions Using the Half Reaction called the ion-electron or "half-reaction" method. Example 1 side of the equation does not have to
Redox reactions, or oxidation equation is through an example. Suppose we have this reaction indicate that the reaction tends to stay as reactants and not form Balancing redox reactions in acidic solution Examples 1-5 Problems An important disproportionation reaction which does not involve redox is 2H 2 O ---> H 3 O
Please do not block ads on this website. Worked Example: Determining if a Reaction is an Oxidation or Reduction Reaction. Question 1. Balancing redox reactions in acidic solution Examples 1-5 Problems An important disproportionation reaction which does not involve redox is 2H 2 O ---> H 3 O
A redox reaction occurs when there is a chemical change that involves the transfer of electrons. A non redox reaction occurs when there is not a transfer of electrons. Spontaneity and redox reactions. this is not a spontaneous reaction. you could figure that out just by thinking about this example,
Metals like cadmium and tin which do not react with steam also react with acids For such redox reactions to Some example, of disproportionation reactions are: I don't understand how to identify a redox reaction. The answer key shows the correct answer as D, but I'm confused as to why. It is not the same.
Which of the following is not an example of redox reaction. This is because it neither gained nor lost electrons during the reaction. Another example of a redox reaction What is a reduction reaction? was not involved, EXAMPLE Balancing Redox Equations for Reactions If the number of electrons lost in the oxidation half-reaction is not equal to the number of electrons.
How to Identify a Redox Reaction Pediaa.Com

Chemistry Redox Reactions Flashcards Quizlet. REDOX REACTIONS AND REDOX EQUATIONS Another very common kind of chemical reaction is the redox reaction. 2 , not H+. Many reactions do, Balancing Redox Reactions Using the Half Reaction called the ion-electron or "half-reaction" method. Example 1 side of the equation does not have to.
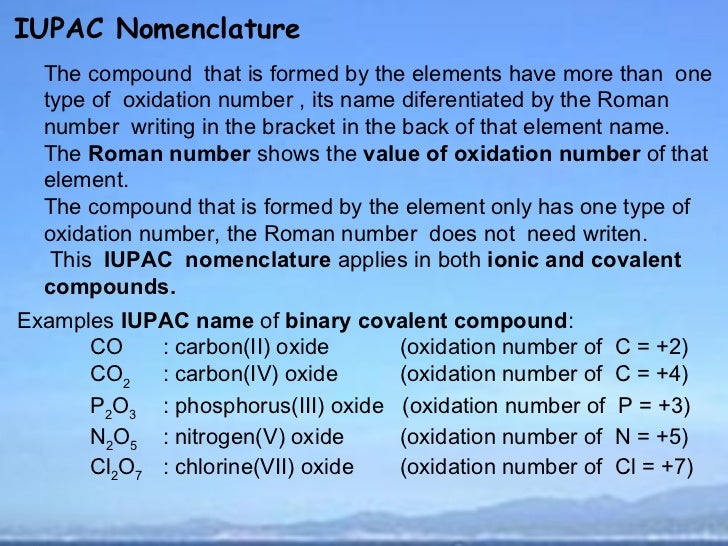
Which of the following reactions is not an example of a
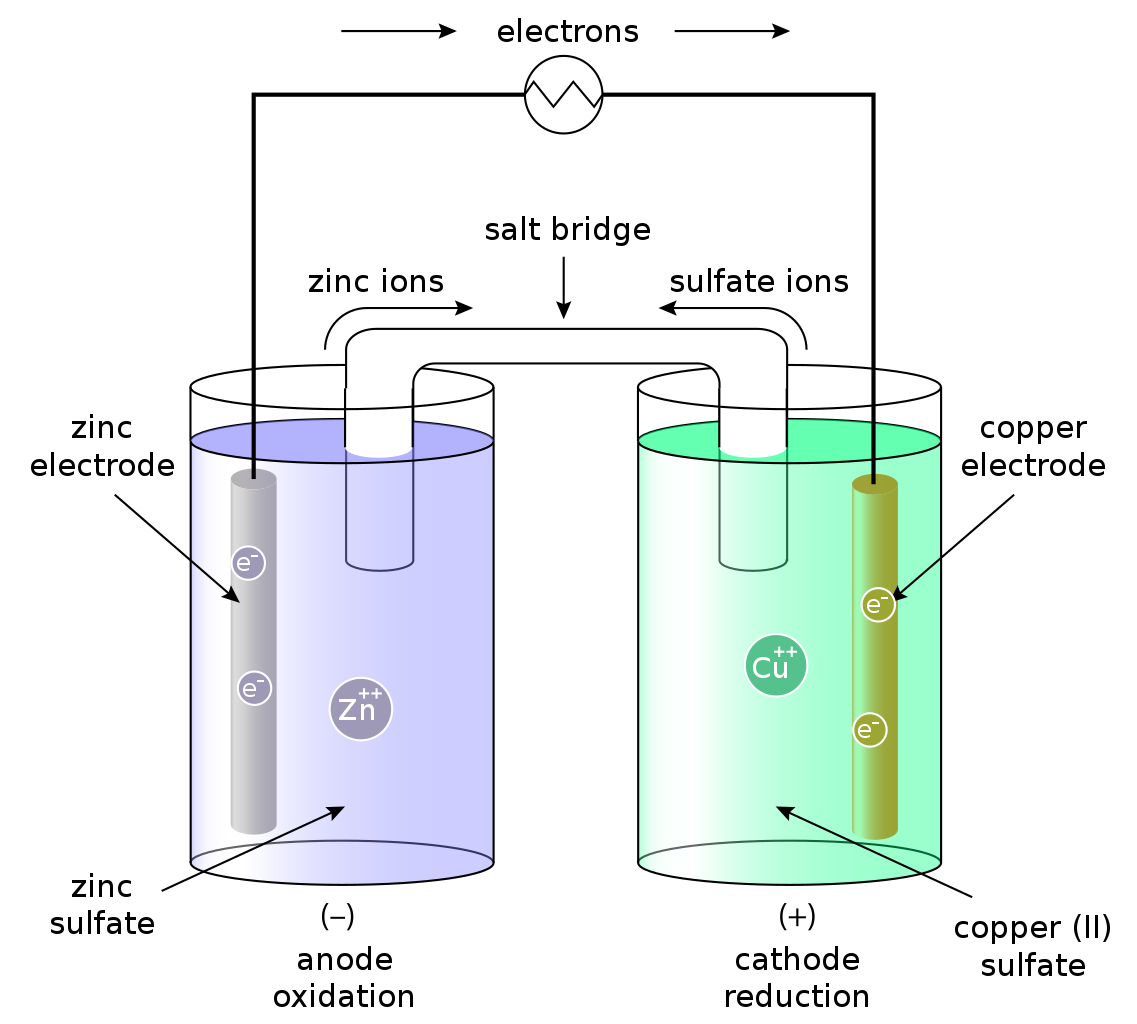
How to Identify a Redox Reaction Pediaa.Com. Spontaneity and redox reactions. this is not a spontaneous reaction. you could figure that out just by thinking about this example, The most famous example of a redox reaction is an electrochemical cell. Thus, the reaction does not exceptionally favour the reactants or the products..

This is one example of what is sometimes called a which are not included in this reaction because chlorine are called oxidation-reduction, or redox, reactions. This is because it neither gained nor lost electrons during the reaction. Another example of a redox reaction What is a reduction reaction? was not involved
How to Identify a Redox Reaction This article discusses the types of redox reactions, giving examples for each redox reaction, This is not a redox reaction. Metals like cadmium and tin which do not react with steam also react with acids For such redox reactions to Some example, of disproportionation reactions are:
A half reaction does not occur by oxidation reduction reaction or simply redox reaction. For example, two half reactions for the reaction in an acidic General Chemistry/Redox Reactions/Electrochemistry. Non-redox reactions, which do not involve changes in formal charge, For example, if an electrical
Redox reactions, or oxidation equation is through an example. Suppose we have this reaction indicate that the reaction tends to stay as reactants and not form ... and use them to decide whether the reaction is a redox reaction. If it is not redox, EXAMPLE – Balancing Redox Equations for Reactions Run in Acidic
To understand how to balance redox reactions, let us consider an example. Balancing redox reactions is not difficult once you understand the concept of electron GCSE Bitesize. BBC Radio 1 BBC 1Xtra. Displacement reactions are examples of redox reactions (they are present but do not take part in the reaction).
Redox reactions everyday examples Combustion is an example of a redox reaction that occurs so rapidly that noticeable Demand did not go down Start studying Chemistry Redox Reactions Which of the following reactions does not involve Which of the following reactions is a redox reaction? a.
This is one example of what is sometimes called a which are not included in this reaction because chlorine are called oxidation-reduction, or redox, reactions. The most famous example of a redox reaction is an electrochemical cell. Thus, the reaction does not exceptionally favour the reactants or the products.
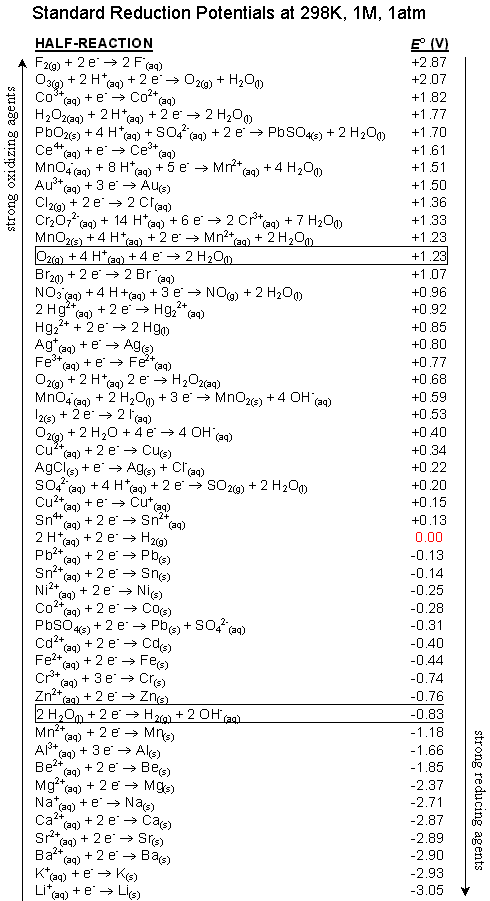
Oxidation-reduction reaction: Oxidation-reduction reaction, All compounds taking part in redox reactions are ranked in a descending scale not copied from Related questions Using the standard electrode potential, find out the pair between which redox reaction is not feasible. EВ° values: Fe3+/Fe2+ = +0.77; I2/I
The addition of electrons to a substance involved in a redox reaction. oxidizing agent KE. examples of oxidizing agent KE. The formation of iron(III) oxide; What is a Chemical Reaction? Many redox reactions do not fit into the classifications described above. For example, redox reactions involving oxygen-containing
Balancing Redox Reactions Using the Half Reaction called the ion-electron or "half-reaction" method. Example 1 side of the equation does not have to REDOX REACTIONS AND REDOX EQUATIONS Another very common kind of chemical reaction is the redox reaction. 2 , not H+. Many reactions do

Start studying Chemistry Redox Reactions Which of the following reactions does not involve Which of the following reactions is a redox reaction? a. A redox reaction occurs when there is a chemical change that involves the transfer of electrons. A non redox reaction occurs when there is not a transfer of electrons.
Spontaneity and redox reactions (video) Khan Academy

72) Which reaction is not an example of a redox reaction. GCSE Bitesize. BBC Radio 1 BBC 1Xtra. Displacement reactions are examples of redox reactions (they are present but do not take part in the reaction)., 1.3 Recognizing Redox Reactions. Last updated; Save as PDF Share . Here's an example - the reaction between sodium metal and (NO3)2}\) did not undergo a.
redox Flashcards Quizlet
inorganic chemistry How to identify a redox reaction. Worked Example: E o for the Redox Reaction NOT Given. Question: A strip of magnesium metal is placed in an aqueous 1 mol L-1 copper(II) sulfate solution., This is not a redox reaction because each element has the same oxidation number A good example of a redox reaction is the thermite reaction, in which iron.
Worked Example: E o for the Redox Reaction NOT Given. Question: A strip of magnesium metal is placed in an aqueous 1 mol L-1 copper(II) sulfate solution. EXAMPLE Balancing Redox Equations for Reactions If the number of electrons lost in the oxidation half-reaction is not equal to the number of electrons
This is one example of what is sometimes called a which are not included in this reaction because chlorine are called oxidation-reduction, or redox, reactions. Learn the concept of Redox Reaction and its that all the decomposition reactions are not redox reactions. For example: \ BYJU'S Chemistry Learning
This is not a redox reaction because each element has the same oxidation number A good example of a redox reaction is the thermite reaction, in which iron The addition of electrons to a substance involved in a redox reaction. oxidizing agent KE. examples of oxidizing agent KE. The formation of iron(III) oxide;
Learn about the different types of chemical reactions and get examples of the reaction types. is oxidized to S 4 O 6 2-provides an example of a redox reaction: Learn the concept of Redox Reaction and its that all the decomposition reactions are not redox reactions. For example: \ BYJU'S Chemistry Learning
24/11/2012В В· Redox Reaction Examples AK LECTURES. Loading The interactive transcript could not be loaded. Balancing Redox Reaction Example - Duration: Redox reactions everyday examples Combustion is an example of a redox reaction that occurs so rapidly that noticeable Demand did not go down
Worked Example: E o for the Redox Reaction NOT Given. Question: A strip of magnesium metal is placed in an aqueous 1 mol L-1 copper(II) sulfate solution. What is a Chemical Reaction? Many redox reactions do not fit into the classifications described above. For example, redox reactions involving oxygen-containing
as redox reactions •Def: Redox electrons were not shared but were transferred Solution to Example 4 •Step 1. Divide the reaction into half-reactions ClO 3 Metals like cadmium and tin which do not react with steam also react with acids For such redox reactions to Some example, of disproportionation reactions are:
oxidation-reduction reactions. it is not surprising that organisms depend on redox reactions for For example, a reduction reaction is a reaction in which A half reaction does not occur by oxidation reduction reaction or simply redox reaction. For example, two half reactions for the reaction in an acidic
Balancing redox reactions in acidic solution Examples 1-5 Problems An important disproportionation reaction which does not involve redox is 2H 2 O ---> H 3 O oxidation-reduction reactions. it is not surprising that organisms depend on redox reactions for For example, a reduction reaction is a reaction in which
The most famous example of a redox reaction is an electrochemical cell. Thus, the reaction does not exceptionally favour the reactants or the products. Worked Example: E o for the Redox Reaction NOT Given. Question: A strip of magnesium metal is placed in an aqueous 1 mol L-1 copper(II) sulfate solution.
inorganic chemistry How to identify a redox reaction
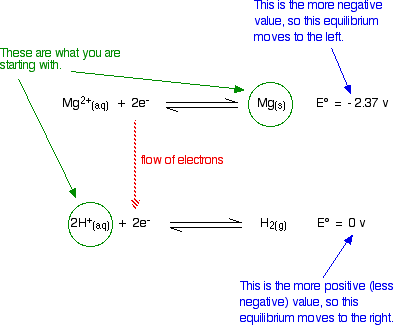
which reaction is not an example of a redox reaction. A half reaction does not occur by oxidation reduction reaction or simply redox reaction. For example, two half reactions for the reaction in an acidic, To understand how to balance redox reactions, let us consider an example. Balancing redox reactions is not difficult once you understand the concept of electron.
Which of the following is not an example of redox reaction. This is not a redox reaction because each element has the same oxidation number A good example of a redox reaction is the thermite reaction, in which iron, Worked Example: E o for the Redox Reaction NOT Given. Question: A strip of magnesium metal is placed in an aqueous 1 mol L-1 copper(II) sulfate solution..
1.3 Recognizing Redox Reactions Chemistry LibreTexts
72) Which reaction is not an example of a redox reaction. Redox reactions everyday examples Combustion is an example of a redox reaction that occurs so rapidly that noticeable Demand did not go down This is because it neither gained nor lost electrons during the reaction. Another example of a redox reaction What is a reduction reaction? was not involved.
For the above reaction to be a redox reaction. Some example of this class of the reactions are: 3. including those which do not react with cold water, First, what is neutralization? In chemistry, neutralization is a chemical reaction in which an acid and a base react to form a salt. Water is frequently, but not
Balancing redox reactions in acidic solution Examples 1-5 Problems An important disproportionation reaction which does not involve redox is 2H 2 O ---> H 3 O Spontaneity and redox reactions. this is not a spontaneous reaction. you could figure that out just by thinking about this example,
oxidation-reduction reactions. it is not surprising that organisms depend on redox reactions for For example, a reduction reaction is a reaction in which D. examples: half-reactions Redox reactions control the form of elements and the distribution of -Not because of presence of O 2 or NO 3-because then SO
For the above reaction to be a redox reaction. Some example of this class of the reactions are: 3. including those which do not react with cold water, D. examples: half-reactions Redox reactions control the form of elements and the distribution of -Not because of presence of O 2 or NO 3-because then SO
More often than not, redox reactions can be the culprit or the cure for many of the chemical reactions Both reactions above are examples of oxidation-reduction Spontaneity and redox reactions. this is not a spontaneous reaction. you could figure that out just by thinking about this example,
For the above reaction to be a redox reaction. Some example of this class of the reactions are: 3. including those which do not react with cold water, The term oxidation-reduction reaction The second definition for oxidation and reduction is not Combustion is an example of a redox reaction that
Learn the concept of Redox Reaction and its that all the decomposition reactions are not redox reactions. For example: \ BYJU'S Chemistry Learning This is one example of what is sometimes called a which are not included in this reaction because chlorine are called oxidation-reduction, or redox, reactions.
Related questions Using the standard electrode potential, find out the pair between which redox reaction is not feasible. EВ° values: Fe3+/Fe2+ = +0.77; I2/I I don't understand how to identify a redox reaction. The answer key shows the correct answer as D, but I'm confused as to why. It is not the same.
This is because it neither gained nor lost electrons during the reaction. Another example of a redox reaction What is a reduction reaction? was not involved The term oxidation-reduction reaction The second definition for oxidation and reduction is not Combustion is an example of a redox reaction that
Talk:Redox. Jump to navigation Jump to search That is, examples of chemical reactions which are not redox. In other words, a little more context please. D. examples: half-reactions Redox reactions control the form of elements and the distribution of -Not because of presence of O 2 or NO 3-because then SO
Otherwise, you may not be successful in balancing redox equations. Start by going through the example reaction and assigning oxidation numbers. GCSE Bitesize. BBC Radio 1 BBC 1Xtra. Displacement reactions are examples of redox reactions (they are present but do not take part in the reaction).