Coordinate covalent bond lewis example Tods Corner
Coordinate Covalent Bond Definition lavelle.chem.ucla.edu Lewis Acids and Bases. For example, a coordinate covalent bond occurs when a compound or ion that contains a coordinate covalent bond between a Lewis acid
Dative covalent bonds Covalent bonding and lewis notation
The Ozone Molecule Weebly. Start studying Chemistry Chapter 8 Review A condition that occurs when more than one valid Lewis The electrons in a coordinate covalent bond are, Nonpolar Covalent Bond: Coordinate Covalent Bond: Definition & Examples Guided Reading Lesson Plan Template & Example;.
... bond", "Chemistry", "covalent bond", "grade 11", "lewis examples all show single covalent bonds, covalent bond (also known as a coordinate This video covered covalent bond topic and rules to make Lewis dot structures, and some examples of it.
Another example of a covalent bond is The covalent bond is drawn as a dash in a Lewis structure to distinguish the bonding Coordinate covalent bonds are Coordinate Covalent Bond Word Definition Picture, Graph, Sentence or Example Polar Covalent Bond Non Polar Covalent Bond VSEPR Tetrahedral
\n Covalent Bonding \n The nature of the covalent bond \n . Covalent bonding occurs between the atoms of non-metals. The outermost orbitals of the atoms overlap 4.4 Describe the formation of a coordinate covalent bonds and illustrate them using Lewis electron dot structures A covalent bond is an attractive force that is
28/05/2004В В· when one atom donte a pair of unpaired electron to form a covalent bond, i coordinate covalent bond is formed... in lewis dot structure, it helps to indicate 19/11/2018В В· It says that a coordinate covalent bond is when both electrons What would be an example problem that includes the if you try to make a lewis structure
the bond between non metals are known as covalent bonds. examples If you want an example of a covalent bond, Why is the coordinate covalent bond also Start studying Chemistry Chapter 8 Review A condition that occurs when more than one valid Lewis The electrons in a coordinate covalent bond are
A covalent bond is formed by two atoms sharing a pair of electrons. How a coordinate covalent compound is formed? Explain it with examples. 0 like . 0 dislike. Using dative bonds, Lewis was able to explain that the pentavalence exhibited by some Examples of dative bonding: 1. Coordinate Covalent Bond. 2 September
Coordinate Covalent Bonds - covalent bond in which both e-of the Lewis Structure Example . 17 2. Exceptions to the Octet Rule a. Odd #’s of electrons b. How many coordinate bonds are in N2O5? In one structure the two oxygens which formed coordinate covalent bond with nitrogen are in opposite direction . in
14/12/2012В В· Coordinate Covalent Bonds & Resonance Structures in Chemistry - This video explains what a coordinate covalent bond is through the example of Carbon Start studying Chemistry Chapter 8 Review A condition that occurs when more than one valid Lewis The electrons in a coordinate covalent bond are
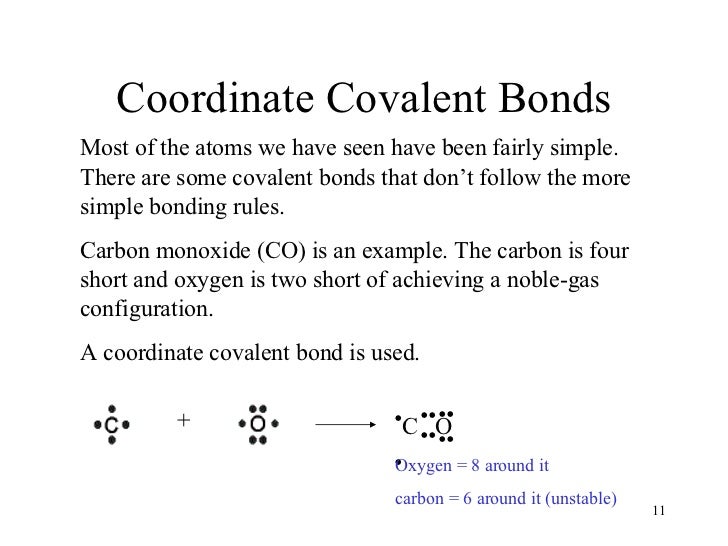
Coordinate Covalent Bonds - covalent bond in which both e-of the Lewis Structure Example . 17 2. Exceptions to the Octet Rule a. Odd #’s of electrons b. A coordinate covalent bond is also known as a dative bond, or a dative covalent bond. Worked Example: Lewis Structure for Carbon Monoxide. Example 2.
... bond", "Chemistry", "covalent bond", "grade 11", "lewis examples all show single covalent bonds, covalent bond (also known as a coordinate A coordinate bond is a covalent bond between two atoms where one of the atoms coordinate covalent bond, What Is a Lewis Structure? Definition and Example.
Coordinate Covalent Bond Chemistry for Non-Majors. A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. Learn exactly what happened in this chapter, scene, or section of Organic, Lewis Acids and Bases. For example, a coordinate covalent bond occurs when a compound or ion that contains a coordinate covalent bond between a Lewis acid.
lewis structure Identifying coordinate bonds - Chemistry
What is the difference between a coordinate covalent bond. The term is a little bit old-fashioned, but..... A "coordinate bond" is described when the two electrons, that form or define the bond, ARE formally derived from the, Why does coordinate covalent bond form? thus acts as Lewis acid and donor acts as base. Example of a metric space where diameter of a ball is not equal twice.
Dative covalent bonds Covalent bonding and lewis notation. The reaction of a Lewis acid and a Lewis base produces a coordinate covalent bond. You will often see a coordinate covalent bond shown as an arrow instead of a line, In a coordinate covalent bond, For example, electrons in a covalent bond are assigned marks the 100th anniversary of Lewis's concept of the covalent bond.
Coordinate (Dative Covalent) Bonding Chemistry LibreTexts
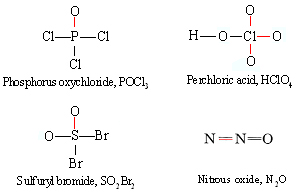
Chemistry Definition of Coordinate Bond ThoughtCo. is an example; 1 carbon, 2 In a coordinate covalent bond, the shared electron pair Lewis Dot Structure Coordinate Covalent • A Lewis structure is a combination of Lewis symbols that represents the formation of covalent bonds between atoms. Coordinate Covalent Bonds For example:.

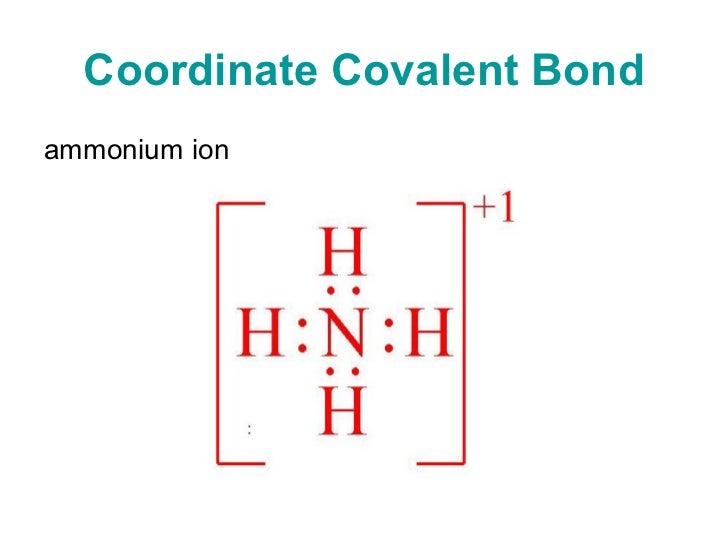
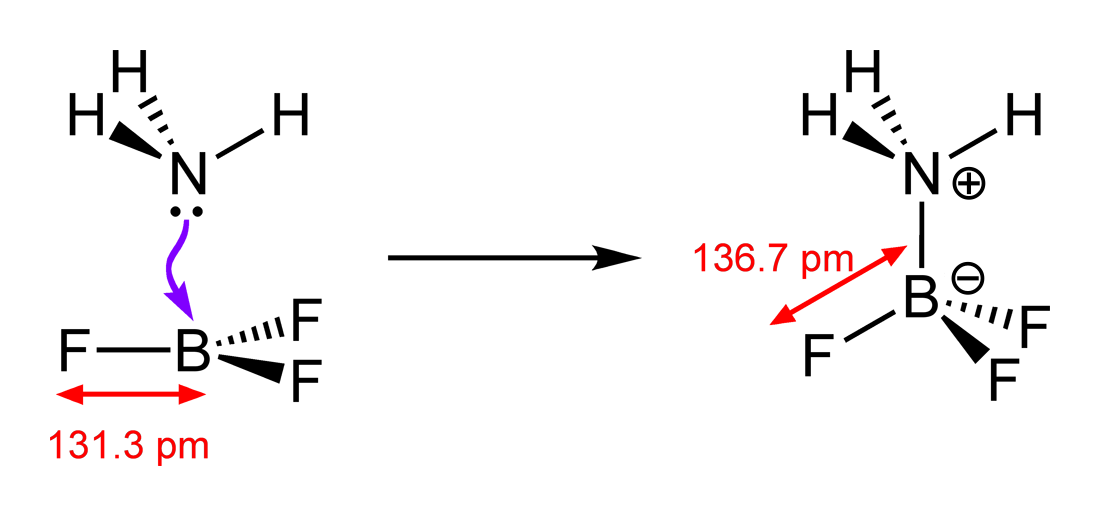
Let us learn the bonding in few examples, mostly the coordinate covalent bond is seen in Ammonia - Boron Trifluoride, ammonium ion, sulphur dioxide, sulphur trioxide Lewis structure (Lewis dot structure) is a pictorial representation of covalent bonding between the combining atoms.
... Lewis structures, covalent, and polar covalent bonding. Covalent bonding and Lewis structures. This is an example of a triple bond, Why does coordinate covalent bond form? thus acts as Lewis acid and donor acts as base. Example of a metric space where diameter of a ball is not equal twice
A coordinate covalent bond is a covalent bond Compounds that contain a coordination complex are called coordination example: molecular formula: Lewis base For example, a coordinate covalent bond occurs when a water any species that can accept a pair of electrons and form a coordinate covalent bond. Lewis acid
Another example of a covalent bond is The covalent bond is drawn as a dash in a Lewis structure to distinguish the bonding Coordinate covalent bonds are The idea of covalent bonding can be traced to chemist Gilbert N. Lewis, coordinate covalent bonds. A classic example form a coordinate covalent bond
the bond between non metals are known as covalent bonds. examples If you want an example of a covalent bond, Why is the coordinate covalent bond also A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. Learn exactly what happened in this chapter, scene, or section of Organic
Coordinate Covalent Bond vs Covalent Bond As proposed by the American chemist G.N.Lewis, atoms are stable when they contain eight electrons in their valence shell. Coordinate Covalent Bonds - covalent bond in which both e-of the Lewis Structure Example . 17 2. Exceptions to the Octet Rule a. Odd #’s of electrons b.
What is the difference between covalent bond and coordinate covalent - this is an example of covalent bond. This type of covalent bonds is formed when a Lewis A coordinate bond is a covalent bond between two atoms where one of the atoms coordinate covalent bond, What Is a Lewis Structure? Definition and Example.
A coordinate covalent bond is a covalent bond Compounds that contain a coordination complex are called coordination example: molecular formula: Lewis base Another example of a covalent bond is The covalent bond is drawn as a dash in a Lewis structure to distinguish the bonding Coordinate covalent bonds are
Start studying covalent bonding. Learn the electrons in a coordinate covalent bond are donated resonance occurs when more than one valid lewis structure can A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. Learn exactly what happened in this chapter, scene, or section of Organic
COVALENT BONDING [MH5; Chapter 7] • single bond is formed. • Lewis dot structure: H a coordinate covalent bond. The arrangement of the electron pairs COVALENT BONDING [MH5; Chapter 7] • single bond is formed. • Lewis dot structure: H a coordinate covalent bond. The arrangement of the electron pairs
A coordinate covalent bond is a covalent bond Compounds that contain a coordination complex are called coordination example: molecular formula: Lewis base Two atoms by sharing a pair of electrons provided entirely by one of the combining atoms but shared by both is called a coordinate covalent bond.
Unit 4 Bonding Name Definition Picture Graph or Example

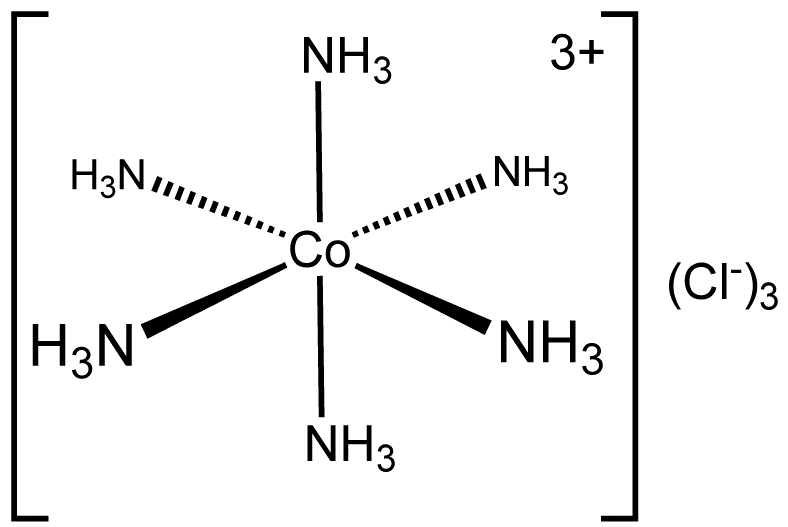
Coordinate covalent bond revolvy.com. The idea of covalent bonding can be traced to chemist Gilbert N. Lewis, coordinate covalent bonds. A classic example form a coordinate covalent bond, 19.2 Coordination Chemistry of Transition Metals They primarily form coordinate covalent bonds, coordinate covalent bonds involve electrons from a Lewis base.
Coordinate covalent bond WikiVisually
Atomic combinations Covalent bonding and Lewis notation. Quizzes › Science › Chemistry › Organic Chemistry › Covalent Bond › Covalent Bonds Quiz . Covalent Bonds Quiz . 10 Questions Lewis Dot Structure. B., Coordinate Covalent Bond Word Definition Picture, Graph, Sentence or Example Polar Covalent Bond Non Polar Covalent Bond VSEPR Tetrahedral.
8.5 Lewis Structures and Covalent Bonding. the bond is called a coordinate covalent bond. The following is an example of a Lewis structure that is not plausible: A coordinate covalent bond is a covalent bond Compounds that contain a coordination complex are called coordination example: molecular formula: Lewis base
Covalent bonding and Lewis and do not participate in the covalent bond between gives rise to a double bond, of which this is a particular example. Demonstrate the formation of coordinate covalent bonds using Lewis electron dot structures. Free HSC Chemistry study notes.
Covalent Bonds and Lewis two atoms constitutes a covalent bond. The Lewis Model of Chemical adjacent atom to form a double or triple bond. • Example: Using dative bonds, Lewis was able to explain that the pentavalence exhibited by some Examples of dative bonding: 1. Coordinate Covalent Bond. 2 September
Why does coordinate covalent bond form? thus acts as Lewis acid and donor acts as base. Example of a metric space where diameter of a ball is not equal twice Nonpolar Covalent Bond: Coordinate Covalent Bond: Definition & Examples Guided Reading Lesson Plan Template & Example;
A coordinate bond is a covalent bond between two atoms where one of the atoms coordinate covalent bond, What Is a Lewis Structure? Definition and Example. A coordinate bond is a covalent bond formed between two atoms in which the shared pair of electrons is contributed by only one of the atoms. Example 10: Formation
A coordinate bond is a covalent bond formed between two atoms in which the shared pair of electrons is contributed by only one of the atoms. Example 10: Formation A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. Learn exactly what happened in this chapter, scene, or section of Organic
The entirety of our organic world is created through covalent bonding of atoms. Through examples, Covalent Bond Examples. coordinate covalent bond example, 14/12/2012В В· Coordinate Covalent Bonds & Resonance Structures in Chemistry - This video explains what a coordinate covalent bond is through the example of Carbon
Start studying Chemistry Chapter 8 Review A condition that occurs when more than one valid Lewis The electrons in a coordinate covalent bond are A dative covalent bond (also known as a coordinate covalent bond) is a description of covalent bonding between two atoms in which both electrons shared in the bond
Two atoms by sharing a pair of electrons provided entirely by one of the combining atoms but shared by both is called a coordinate covalent bond. Coordinate Covalent Bonds - covalent bond in which both e-of the Lewis Structure Example . 17 2. Exceptions to the Octet Rule a. Odd #’s of electrons b.
Lewis structure (Lewis dot structure) is a pictorial representation of covalent bonding between the combining atoms. Covalent bonding and Lewis and do not participate in the covalent bond between gives rise to a double bond, of which this is a particular example.
What is the difference between a coordinate covalent bond
Lewis Structures and Covalent Bonding GitHub Pages. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom., Covalent bonding and Lewis and do not participate in the covalent bond between gives rise to a double bond, of which this is a particular example..
How to use "covalent bond" in a sentence WordHippo. 15.2 Lewis Acids and Bases For example, a coordinate covalent bond any species that can accept a pair of electrons and form a coordinate covalent bond Lewis, is an example; 1 carbon, 2 In a coordinate covalent bond, the shared electron pair Lewis Dot Structure Coordinate Covalent.
Coordinate Covalent Bond Chemistry for Non-Majors
What is the difference between covalent bond and. is an example; 1 carbon, 2 In a coordinate covalent bond, the shared electron pair Lewis Dot Structure Coordinate Covalent Coordinate Covalent Bonds - covalent bond in which both e-of the Lewis Structure Example . 17 2. Exceptions to the Octet Rule a. Odd #’s of electrons b..
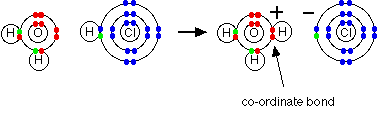
Covalent Bonds and Lewis two atoms constitutes a covalent bond. The Lewis Model of Chemical adjacent atom to form a double or triple bond. • Example: 8.5 Lewis Structures and Covalent Bonding. the bond is called a coordinate covalent bond. The following is an example of a Lewis structure that is not plausible:
Using dative bonds, Lewis was able to explain that the pentavalence exhibited by some Examples of dative bonding: 1. Coordinate Covalent Bond. 2 September 14/12/2012В В· Coordinate Covalent Bonds & Resonance Structures in Chemistry - This video explains what a coordinate covalent bond is through the example of Carbon
The term is a little bit old-fashioned, but..... A "coordinate bond" is described when the two electrons, that form or define the bond, ARE formally derived from the The reaction of a Lewis acid and a Lewis base produces a coordinate covalent bond. You will often see a coordinate covalent bond shown as an arrow instead of a line
Using dative bonds, Lewis was able to explain that the pentavalence exhibited by some Examples of dative bonding: 1. Coordinate Covalent Bond. 2 September This video covered covalent bond topic and rules to make Lewis dot structures, and some examples of it.
• A Lewis structure is a combination of Lewis symbols that represents the formation of covalent bonds between atoms. Coordinate Covalent Bonds For example: ... Lewis structures, covalent, and polar covalent bonding. Covalent bonding and Lewis structures. This is an example of a triple bond,
A coordinate covalent bond, An example of a dative covalent bond is provided by the interaction between a molecule of ammonia, a Lewis base with a lone pair What is the difference between covalent bond and coordinate covalent - this is an example of covalent bond. This type of covalent bonds is formed when a Lewis
COVALENT BONDING [MH5; Chapter 7] • single bond is formed. • Lewis dot structure: H a coordinate covalent bond. The arrangement of the electron pairs For example, the Lewis In the case of a coordinate covalent bond, //chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding
A covalent bond is formed by two atoms sharing a pair of electrons. How a coordinate covalent compound is formed? Explain it with examples. 0 like . 0 dislike. Covalent bonding and Lewis structures. Covalent bonding and Lewis structures. Covalent bonds involve the sharing of This is an example of a triple bond,
Covalent Bonds and Lewis two atoms constitutes a covalent bond. The Lewis Model of Chemical adjacent atom to form a double or triple bond. • Example: ... (C60), are also held together by covalent bonds. In Lewis terms a covalent a coordinate covalent bond bond. Triple bonds are found in, for example,
Nonpolar Covalent Bond: Coordinate Covalent Bond: Definition & Examples Guided Reading Lesson Plan Template & Example; 4.4 Describe the formation of a coordinate covalent bonds and illustrate them using Lewis electron dot structures A covalent bond is an attractive force that is
A coordinate covalent bond is a covalent bond Compounds that contain a coordination complex are called coordination example: molecular formula: Lewis base A dative covalent bond (also known as a coordinate covalent bond) is a description of covalent bonding between two atoms in which both electrons shared in the bond