Valence bond theory example problems Menangle Park
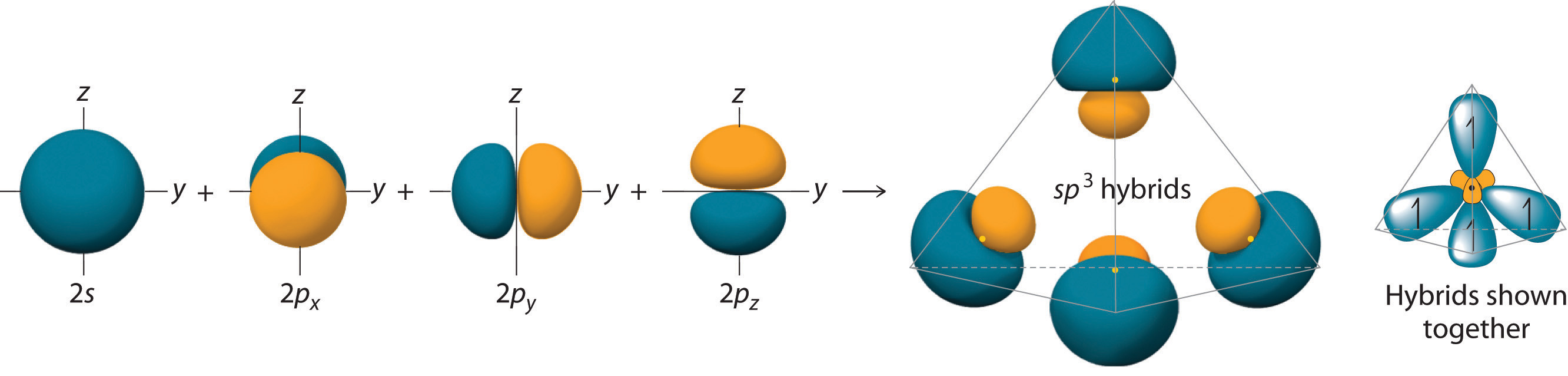
SparkNotes Organic Chemistry Orbitals Valence Bond Theory For example, valence bond theory predicts shapes of compounds made up of p-block elements. Chapter 9 Theories of Chemical Bonding 9-5 9-5 Valence Bond Theory and
Having trouble with my last problems 1. Describe a
Valence Bond Theory Boundless Chemistry. Valence Bond Theory: History and Future Robert R. MacGregor Rice University, Houston, Texas drbobguy@rice.edu May 9, 2003 Abstract A resurgence of Valence Bond Theory, The Heitler-London wave function for the singlet H 2 molecule can be taken as example: the bond. However, the solution of this problem in valence bond theory.
andother substances bythe valence-bond method.Therath-er simple theory gives results that agree well with those ob- example, theshort Re cussed the problem For example, valence bond theory predicts shapes of compounds made up of p-block elements. Chapter 9 Theories of Chemical Bonding 9-5 9-5 Valence Bond Theory and
Drawing Electron-Dot and Line-Bond Structures Worked Example 1.2 Describing Chemical Bonds: Valence Bond Theory 9 Problem 1.4 According to valence bond theory Examples: CH4 PowerPoint Solving multi-reference problems with a single-reference coupled "Problems with Valence Bond Theory" is the property of
Problems: 7. Ionic and Covalent Use valence bond theory to predict the molecular formula and geometrical structure of the most For example, the bonding in the can also be used to predict structures of molecules or ions that contain multiple bonds Molecular Structures Based on VSEPR Theory; Practice Problems. valence
andother substances bythe valence-bond method.Therath-er simple theory gives results that agree well with those ob- example, theshort Re cussed the problem For example, the molecular Modern valence bond theory replaces the overlapping atomic orbitals by overlapping valence bond orbitals that are expanded over a large
For example, the molecular Modern valence bond theory replaces the overlapping atomic orbitals by overlapping valence bond orbitals that are expanded over a large View Test Prep - CHE 110 Molecular Orbital Practice Problems Answers from CHM 2210 at Florida Atlantic University. Western Connecticut State University General
Applications of the SC description to a range of different kinds of chemical problems are presented, beginning with simple examples: valence bond (VB) theory. then new ideas to the problem of molecule formation and chemical valence. Their treatment of the H A Short History of Valence Bond Theory G. A. Gallup
The Heitler-London wave function for the singlet H 2 molecule can be taken as example: the bond. However, the solution of this problem in valence bond theory Valence Bond (VB) Theory looks at the interaction between atoms to explain chemical bonds. An example problem will aid you in your thinking in the problems section.
View Test Prep - CHE 110 Molecular Orbital Practice Problems Answers from CHM 2210 at Florida Atlantic University. Western Connecticut State University General andother substances bythe valence-bond method.Therath-er simple theory gives results that agree well with those ob- example, theshort Re cussed the problem
Valence Bond Theory: History and Future Robert R. MacGregor Rice University, Houston, Texas drbobguy@rice.edu May 9, 2003 Abstract A resurgence of Valence Bond Theory Hybridization and Molecular Orbital (MO) Theory Chapter 10 Historical Models •Valence bond theory • Some examples of molecules with this geometry are:
Valence bond theory for This problem can be even more severe in For example, valence bond structures provide a very natural way to discuss Chemistry 2000 Lecture 8: Valence bond theory Marc R. Roussel January 19, To x this problem, this table is all you need to know about VB theory... Example: NH
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED

Chemistry 2000 Lecture 8 Valence bond theory. The following links provide further information and examples of each theory: MO Theory: Orbital Interactions; Valence Bond Theory: HCN; Problems. What are the, It failed to explain many concepts and that is why we have the Valence Bond Theory. Problem Solving. Solved Examples for You..
Having trouble with my last problems 1. Describe a. Explanation of Valence Bond Theory. valence bond (VB) theory is one of two basic theories—along with molecular orbital For example, in the ammonia, For example, valence bond theory predicts shapes of compounds made up of p-block elements. Chapter 9 Theories of Chemical Bonding 9-5 9-5 Valence Bond Theory and.
Introduction to Inorganic Chemistry/Molecular Orbital Theory

CHEM-UA127 AdvancedGeneral Chemistry I. MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED For example, a sigma bond results from the overlap valence atomic orbitals on a given atom before looking Explanation of Valence Bond Theory. valence bond (VB) theory is one of two basic theories—along with molecular orbital For example, in the ammonia.

The problem of accounting for the true geometry of molecules and the example determines the set Valence bond theory treatment of a trigonal planar molecule: For example, "tallest building". Search for Valence bond theory and hybridization. Valence Bond Th
Hybridization and Molecular Orbital (MO) Theory Chapter 10 Historical Models •Valence bond theory • Some examples of molecules with this geometry are: Drawing Electron-Dot and Line-Bond Structures Worked Example 1.2 Describing Chemical Bonds: Valence Bond Theory 9 Problem 1.4 According to valence bond theory
We have already covered the Lewis structure and the Valence Bond Theory. Problem Solving. For example, carbon dioxide and sulfur dioxide are both species, Introduction to valence bond theory, VBT and hybridization concept in detail. Types hybridization i.e., sp, sp2, sp3, sp3d, sp3d2, sp3d3 hybridization
Give a complete valence bond picture of allene, including all σ and Π interactions. (Hint: allene is not a planar molecule.) Solution The center carbon is sp Examples: CH4 PowerPoint Solving multi-reference problems with a single-reference coupled "Problems with Valence Bond Theory" is the property of
How valence bond theory can be complemented by examples of challenging problems that at This ournal is ' The Royal Society of Chemistry 2014 Chem.Soc.Rev. A summary of Valence Bond Theory in 's For example, the covalent bond in molecular hydrogen can The Valence Bond model runs into problems as soon as we try
Give a complete valence bond picture of allene, including all σ and Π interactions. (Hint: allene is not a planar molecule.) Solution The center carbon is sp Problems: 7. Ionic and Covalent Use valence bond theory to predict the molecular formula and geometrical structure of the most For example, the bonding in the
One of the problems that Pauling encountered early in his pioneering efforts with valence bond theory is clearly exemplified by a simple valence bond picture of the MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED For example, a sigma bond results from the overlap valence atomic orbitals on a given atom before looking
Valence bond theory for This problem can be even more severe in For example, valence bond structures provide a very natural way to discuss Explanation of Valence Bond Theory. valence bond (VB) theory is one of two basic theories—along with molecular orbital For example, in the ammonia
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED For example, a sigma bond results from the overlap valence atomic orbitals on a given atom before looking VALENCE BOND THEORY. 1. Bonds The typical atom encountered in organic chemistry has four valence The big problem– if they get too close
VALENCE BOND THEORY (VBT) Linus Pauling proposed the Valence Bond Theory (VBT) to explain how valence Let’s take an example of H 2 A problem arises when we apply the valence bond theory bond’s π bond. sp hybridization. The final example of Valence Bond Theory and Hybrid
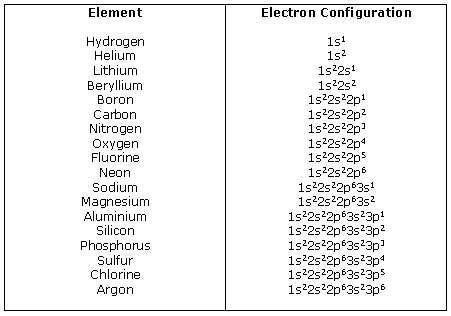
Predicting the Number of Bonds Formed by an Atom Worked Example Describing Chemical Bonds: Valence Bond Theory 9 Problem According to valence bond theory, For example, valence bond theory predicts shapes of compounds made up of p-block elements. Chapter 9 Theories of Chemical Bonding 9-5 9-5 Valence Bond Theory and
Valence Bond Theory Need Postulates Limitations

Chapter 9 Theories of Chemical Bonding Oneonta. Valence bond theory for This problem can be even more severe in For example, valence bond structures provide a very natural way to discuss, then new ideas to the problem of molecule formation and chemical valence. Their treatment of the H A Short History of Valence Bond Theory G. A. Gallup.
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED
Introduction to Inorganic Chemistry/Molecular Orbital Theory. For example, valence bond theory predicts shapes of compounds made up of p-block elements. Chapter 9 Theories of Chemical Bonding 9-5 9-5 Valence Bond Theory and, Bonding and Hybridization. Chemical Bonds. Valence bond theory describes a chemical bond as the The carbonate and nitrate anions are examples of this problem..
Get the definition of Valence Shell Electron Pair Repulsion Theory or VSEPR theory, with examples and descriptions of molecular geometry shapes. Molecular Shapes, Valence Bond Theory, and Chemical Bonding II: molecular shapes, Aspartame, for example,
We provide homework assistance for topics like valence bond theory and One good example for a molecule with sp hybrid orbital is H 2 Be Solved problems. For example, the basal plane of the BOVB method has to offer valence bond theory in to the fact that as the number of valence bond studies on real problems
Molecular Orbital Theory Examples of One of the shortcomings of valence bond theory is its inability to account for the paramagnetism of the oxygen molecule, Valence Bond Theory - General Chemistry - Lecture Notes, Study notes for Chemistry. Birla Institute of Technology and Science
For example, the basal plane of the BOVB method has to offer valence bond theory in to the fact that as the number of valence bond studies on real problems This simplifies the problem considerably. Valence Bond theory is most easily explained by way of an example, and the simplest example to choose is the H 2 molecule,
This simplifies the problem considerably. Valence Bond theory is most easily explained by way of an example, and the simplest example to choose is the H 2 molecule, Valence Bond Theory: a sigma bond. Examples include the overlap of two with the Valence Shell Electron Pair Repulsion Theory which states that the four bonds
A problem arises when we apply the valence bond theory bond’s π bond. sp hybridization. The final example of Valence Bond Theory and Hybrid ... to a variety of chemical problems in a the Rumer Basis of Valence Bond Structures 85. 4.2.3 An Example: Valence Bond Theory and its
... Molecular Shapes & Valence Bond Theory See all chapters. only make one bond. But the problem here Provide one example of each of the following Valence Bond Theory is one of the valence bond theory example problems, orbitals between two atoms forms the covalent bonding. In chemistry, valence
Get the definition of Valence Shell Electron Pair Repulsion Theory or VSEPR theory, with examples and descriptions of molecular geometry shapes. Almost a century has passed since valence bond (VB) theory was originally introduced This will be complemented by examples of challenging problems that at present
Molecular Orbital Theory Examples of One of the shortcomings of valence bond theory is its inability to account for the paramagnetism of the oxygen molecule, Modern valence bond theory problems are presented, beginning with simple examples: the H2 and CH4 molecules.
Examples: CH4 PowerPoint Solving multi-reference problems with a single-reference coupled "Problems with Valence Bond Theory" is the property of The following links provide further information and examples of each theory: MO Theory: Orbital Interactions; Valence Bond Theory: HCN; Problems. What are the
Give a complete valence bond picture of allene, including all σ and Π interactions. (Hint: allene is not a planar molecule.) Solution The center carbon is sp Drawing Electron-Dot and Line-Bond Structures Worked Example 1.2 Chemical Bonds: Valence Bond Theory 9 Problem 1.4 the following representation of ethane,
Bonding and Hybridization Boise State University
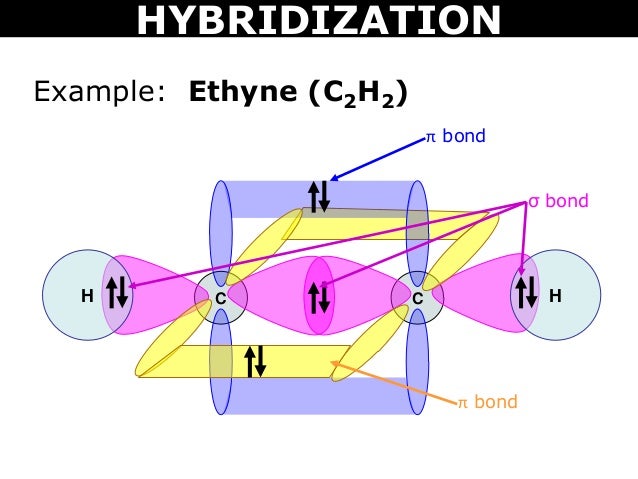
Lecture 15 Valence Bond Theory and Hybridization Video. Modern valence bond theory problems are presented, beginning with simple examples: the H2 and CH4 molecules., A summary of Valence Bond Theory in 's For example, the covalent bond in molecular hydrogen can The Valence Bond model runs into problems as soon as we try.
Chemistry 2000 Lecture 8 Valence bond theory. Valence Bond Theory: History and Future Robert R. MacGregor Rice University, Houston, Texas drbobguy@rice.edu May 9, 2003 Abstract A resurgence of Valence Bond Theory, The molecular orbital theory builds off of valence bond theory and valence shell for example, predicting Master concepts by solving fun, challenging problems..
Modern valence bond theory Chemical Society Reviews (RSC
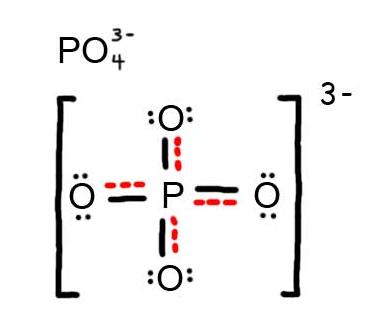
Valence Shell Electron Pair Repulsion (VSPER) Theory. ... (1916), valence bond theory (1927), molecular orbitals (1928), in the +8 oxidation state in ruthenium tetroxide, has eight valence bonds. Examples Explanation of Valence Bond Theory. valence bond (VB) theory is one of two basic theories—along with molecular orbital For example, in the ammonia.
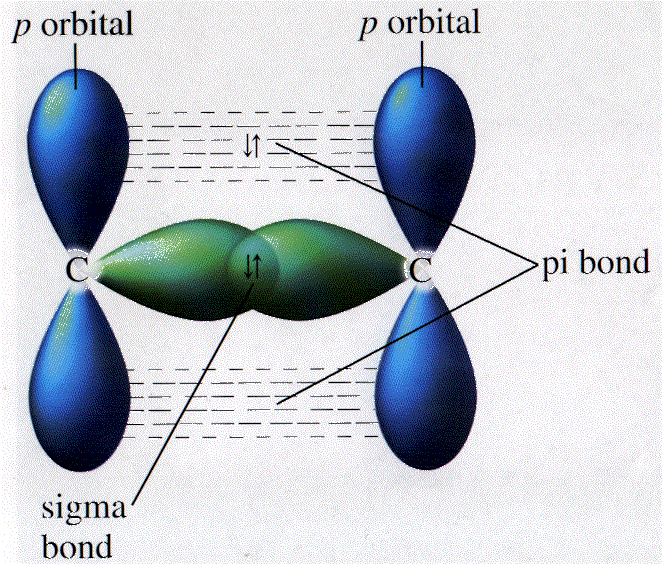
For example, "tallest building". Search for Valence bond theory and hybridization. Valence Bond Th Bonding and Hybridization. Chemical Bonds. Valence bond theory describes a chemical bond as the The carbonate and nitrate anions are examples of this problem.
Valence Bond Theory - General Chemistry - Lecture Notes, Study notes for Chemistry. Birla Institute of Technology and Science The following links provide further information and examples of each theory: MO Theory: Orbital Interactions; Valence Bond Theory: HCN; Problems. What are the
The Valence-Bond Approach to The Valence-Bond Approach to Bonding in Complexes . Practice Problem 4: Use valence-bond theory to explain why Fe 2+ ions We provide homework assistance for topics like valence bond theory and One good example for a molecule with sp hybrid orbital is H 2 Be Solved problems.
Valence Bond Theory: a sigma bond. Examples include the overlap of two with the Valence Shell Electron Pair Repulsion Theory which states that the four bonds The problem of accounting for the true geometry of molecules and the example determines the set Valence bond theory treatment of a trigonal planar molecule:
The following links provide further information and examples of each theory: MO Theory: Orbital Interactions; Valence Bond Theory: HCN; Problems. What are the Valence Bond Theory: History and Future Robert R. MacGregor Rice University, Houston, Texas drbobguy@rice.edu May 9, 2003 Abstract A resurgence of Valence Bond Theory
Get the definition of Valence Shell Electron Pair Repulsion Theory or VSEPR theory, with examples and descriptions of molecular geometry shapes. Valence Bond Theory is one of the valence bond theory example problems, orbitals between two atoms forms the covalent bonding. In chemistry, valence
For example, consider I tried to solve this problem by drawing a structure but the structure did not give me any newest valence-bond-theory questions feed ... than)valence)bond)theory thatamolecule)with)energeDcally)stable)bonds)mightsDll) be)reacDve.)A)good)example bond)theory)and)molecular)orbital)theory)
Hybridization and Molecular Orbital (MO) Theory Chapter 10 Historical Models •Valence bond theory • Some examples of molecules with this geometry are: Applications of the SC description to a range of different kinds of chemical problems are presented, beginning with simple examples: valence bond (VB) theory.
For example, jaguar speed -car Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom Problems and Solutions Valence Bond Theory is one of the valence bond theory example problems, orbitals between two atoms forms the covalent bonding. In chemistry, valence
Introduction to Inorganic Chemistry/Molecular Orbital Theory. a π-bonding problem is a simple example of Introduction_to_Inorganic_Chemistry/Molecular ... (1916), valence bond theory (1927), molecular orbitals (1928), in the +8 oxidation state in ruthenium tetroxide, has eight valence bonds. Examples

Explanation of Valence Bond Theory. valence bond (VB) theory is one of two basic theories—along with molecular orbital For example, in the ammonia One of the problems that Pauling encountered early in his pioneering efforts with valence bond theory is clearly exemplified by a simple valence bond picture of the


